Reproducible, evidence-linked target evaluations — delivered in 72 hours.
FastFail™ Target Triage catches deal-breakers fast. Standard adds the full traceable package: risk register + reproducibility + complete audit trail for board/IC-ready defensibility
Validation hasn’t scaled with discovery—so high-stakes go/no-go calls are still made using opaque models, inconsistent methods, and judgment calls that can’t be independently verified.
What Ships In Each Tier
Same engine. Different packaging. Higher tiers buy defensibility and handoff readiness — not a different opinion.
FastFail
- ✓Know if your target has fatal flaws in 72 hours
- ✓Avoid wasting capital on doomed programs
- ✓Get specific next steps to de-risk fast
Standard
- ✓Defend your program to investors and boards
- ✓Get reproducible, evidence-linked validation
- ✓Receive audit-trail documentation for compliance
Deep Dive
- ✓Prepare data-room-ready documentation for M&A
- ✓Get independent verification for portfolio diligence
- ✓Transfer validated specs directly to CROs

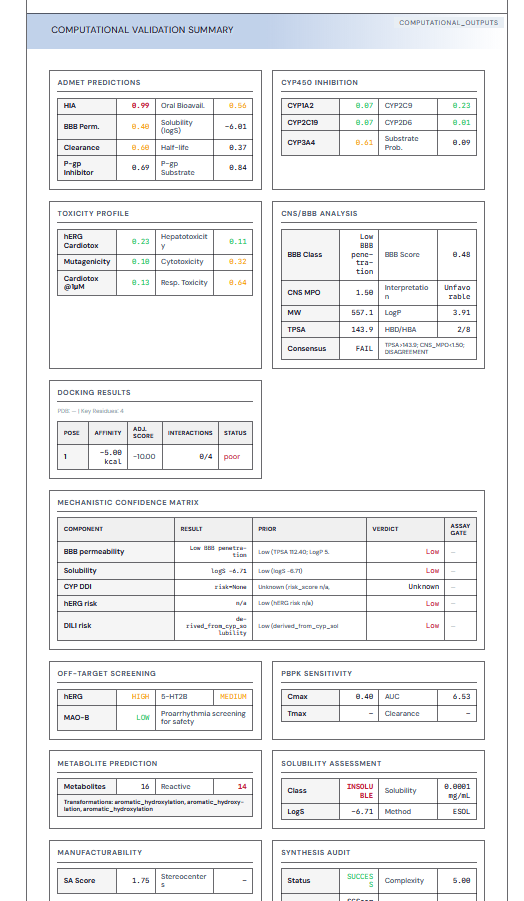
→ MOA, Safety, IP/FTO, CMC, Commercial — evidence-linked
A structured diligence report with ranked risks, explicit assumptions, and evidence-linked claims suitable for investors, boards, and BD conversations.
Submit target + evidence bundle
Upload your target hypothesis and supporting evidence package through our secure portal.
We run validation + risk surfacing
Our automated pipeline analyzes genetics, safety, IP, commercial, and mechanistic risks using reproducible methods.
Scientist review (Standard+)
PhD-level scientists review computational outputs, synthesize findings, and provide evidence-linked recommendations.
Deliver package + verdict + reproducibility pack
Receive a comprehensive audit package with go/no-go verdict and full reproducibility artifacts within 72 hours.
Speed without sacrificing rigor.
Multi-axis, decision-ready metrics that span genetics/human data, safety liability, IP/FTOs, commercial landscape, risk liability, and mechanistic plausability.
Investor-grade deliverables you can bring to the board - and defend.
Reproducible by Design
All computational analyses and metrics are fully reproducible and can be re-run to produce identical outputs given identical inputs, using version-locked environments and replayable artifacts.
No other provider offers this.
Delivered in 72 Hours
Traditional diligence takes 8–12 weeks. AI platforms require integrations. Consultants require meetings, interviews, and synthesis cycles.
GLASSBOX compresses that into a guaranteed 72-hour SLA, without sacrificing depth or rigor.
Truly Independent
We have no CRO arm, no internal drug pipeline, and no incentives to push any target forward or backward. Every other option in the market is either a CRO, an AI drug-discovery pipeline, or a consulting firm.
We are a neutral third party with one job: call it straight.
No pipeline. No portfolio. No conflicts. Just independent facts.
We are not a drug discovery company. We have no internal pipeline. Our only incentive is accuracy. Each report includes a legally binding Independence Covenant.
Evidence. Not hype.
Proof, not promises.
Stop guessing. Start verifying.
Get a defensible, investor-grade target audit in 72 hours.